Showing posts with label Transfer of heat energy. Show all posts
Showing posts with label Transfer of heat energy. Show all posts
Sunday, June 14, 2009
Heat is a type of energy.
Heat energy=Thermal energy
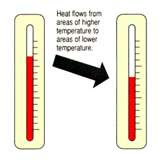
Thermal is the total K.E of the molecules in an object/body. Transfer of thermal energy takes place when there is a difference in temperature, from a higher temperature region to a lower temperature region.
A=50°C B=10°C
When bodies A and B are in contact. A, with a higher temperature than B, would lose heat at a rate faster than the rate at which it absorbs heat from B. While B absorbs heat from A at a rate faster than it loses heat to A. Thus, A loses heat, decrease in temperature, B gains heat, increase in temperature.
Finally, the two bodies would have the same temperature(thermal equilibrium), there is no net flow of thermal energy between them.
Working: (50-10)÷2=20°C
No net flow of thermal energy: 20°C-20°C=0
There are 3 different processes of thermal energy transfer:
1. Conduction, 2. Convection, 3. Radiation

Heat energy=Thermal energy
Thermal is the total K.E of the molecules in an object/body. Transfer of thermal energy takes place when there is a difference in temperature, from a higher temperature region to a lower temperature region.
A=50°C B=10°C
When bodies A and B are in contact. A, with a higher temperature than B, would lose heat at a rate faster than the rate at which it absorbs heat from B. While B absorbs heat from A at a rate faster than it loses heat to A. Thus, A loses heat, decrease in temperature, B gains heat, increase in temperature.
Finally, the two bodies would have the same temperature(thermal equilibrium), there is no net flow of thermal energy between them.
Working: (50-10)÷2=20°C
No net flow of thermal energy: 20°C-20°C=0
There are 3 different processes of thermal energy transfer:
1. Conduction, 2. Convection, 3. Radiation

Subscribe to:
Posts (Atom)